Abstract
Cytogenetic alterations form the basis for risk stratification for multiple myeloma (MM) and guide the selection of therapy; however, current pathology assays performed on bone marrow samples can produce false-negatives due to the unpredictable distribution and rarity of MM cells. Here, we report on a microfluidic device used to facilitate CD45 depletion to enhance the detection of cytogenetic alterations in plasma cells (PCs). Bone marrow samples from 48 patients with MM were each divided into two aliquots. One aliquot was subjected to classic flow cytometry and fluorescent in situ hybridization (FISH). The other first went through CD45+ cell depletion, further enriched by microfluidic size selection. The enriched samples were then analyzed using flow cytometry and FISH and compared to those analyzed using the classic method only. Unlike the traditional method, the microfluidic device removed the CD45+ leukocytes and specifically selected PCs from the remaining white blood cells. Therefore, the microfluidic method (MF-CD45-TACs) significantly increased the percentage of CD38+/CD138+ cells to 37.7 ± 20.4% (P < 0.001) from 10.3 ± 8.5% in bone marrow. After the MF-CD45-TAC enrichment, the detection rate of IgH rearrangement, del(13q14), del(17p), and 1q21 gains, rose to 56.3% (P < 0.001), 37.5% (P < 0.001), 22.9% (P < 0.001), and 41.7% (P = 0.001), respectively; all rates of detection were significantly increased compared to the classically analyzed samples. In this clinical trial, this microfluidic-assisted assay provided a precise detection of cytogenetic alterations in PCs and improved clinical outcomes.
1 Introduction
Multiple myeloma (MM) is an incurable neoplasm of plasma cells (PCs) that affects more than 20 000 people annually in the United States. Risk stratification, primarily based on cytogenetic abnormalities, has emerged as essential for its management (Mikhael et al., 2013). Thalidomide, lenalidomide, and pomalidomide, which represent the first to third generations of immunomodulatory drugs, respectively, are used for MM maintenance therapy. Cytogenetic alterations form the basis of MM risk stratification and selection of immunomodulatory drugs for therapy (Nathwani et al., 2016). PCs undergo clonal evolution: In earlier and smoldering disease, CD45-positive cells predominate, whereas CD45-negative PCs are more prevalent in patients with advanced disease (both new and relapsed) (Kumar et al., 2005). Thus, both CD45− and CD45+ can serve as prognostic biomarkers for MM (Gonsalves et al., 2016) but with distinct prognoses. The tumor load in patients with CD45− cells is higher than those with CD45+ cells, which could be explained by the lower proliferation rate of the latter population (Asosingh et al., 2001). Malignant PCs manifest as CD45− cells with co-expression of CD38/CD138 plus CD19 and CD56, implying the clinical significance of CD45− cells in risk stratification of MM (Langer et al., 2016). Therefore, depletion of CD45+ cells in bone marrow could enrich PCs for this purpose.
Microfluidic device fabrication
Microfluidic DLD devices were fabricated with standard photolithography and soft lithography techniques. Negative photoresist SU8-3025 (Microchem, Westborough, MA, USA) was used to fabricate the master mold on a silicon wafer with a photomask. The patterned silicon wafers were silanized with chlorotrimethylsilane (Aldrich, Burlington, MA, USA) to facilitate particle desorption mass spectrometry mold release. Polydimethylsiloxane (PDMS, RTV615; General Electric, USA) mixed with curing agent (5 : 1 w/w ratio) was poured into the silicon mold and cured at 80 °C for 1 h. Holes were punched for inlet and outlets, and the PDMS mold was bonded to glass slides after oxygen plasma treatment. The design of the DLD device is shown in Fig. 2.
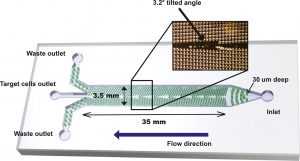
Microfluidic enrichment of plasma cells (MF-CD45-TACs)
The microfluidic DLD design consisted of an inlet, three outlets, and a central flow chamber with micropost array, as shown in Fig. 2. The flow chamber was 35 mm long, 3.5 mm wide, and 30 μm high. The micropost array was tilted at an angle of 3.2° relative to the fluid flow direction. For each patient, a 1.0 mL sample of bone marrow fluid and 500 μL CD45 TACs were mixed at room temperature for 30 min. The mixture was slowly added into 2.0 mL Ficoll in a test tube. Low-speed/low-temperature centrifugation was performed at 450 g for 15 min. The white film layer at the interface of the plasma and the Ficoll solution was transferred to a fresh tube. Then, the white film layer (50–100 μL, 1 × 106 cells) was loaded into DLD chip for PC enrichment. The DLD critical deflection diameter is designed such that large cells (PCs) flow in a bumping mode and thus are concentrated in the center of chamber, while small cells (erythrocytes and most of leukocytes) follow the mainstream direction. We optimized the design in the outlet portion to maximize the PC enrichment. The outlet portion of the device consists of a row of microposts with gradually decreasing gaps. With this design, although part of the erythrocytes and leukocytes escaped through the gaps at the outlet, the PCs can be fully collected once they were concentrated in the DLD chip. The resulting DLD-enriched PCs were then subjected to FACS detection.
Read the original articles:
https://febs.onlinelibrary.wiley.com/doi/10.1002/1878-0261.12201
