Abstract
Brain organoids derived from human pluripotent stem cells provide a highly valuable in vitro model to recapitulate human brain development and neurological diseases. However, the current systems for brain organoid culture require further improvement for the reliable production of high-quality organoids. Here, we demonstrate two engineering elements to improve human brain organoid culture, (1) a human brain extracellular matrix to provide brain-specific cues and (2) a microfluidic device with periodic flow to improve the survival and reduce the variability of organoids. A three-dimensional culture modified with brain extracellular matrix significantly enhanced neurogenesis in developing brain organoids from human induced pluripotent stem cells. Cortical layer development, volumetric augmentation, and electrophysiological function of human brain organoids were further improved in a reproducible manner by dynamic culture in microfluidic chamber devices. Our engineering concept of reconstituting brain-mimetic microenvironments facilitates the development of a reliable culture platform for brain organoids, enabling effective modeling and drug development for human brain diseases.
Introduction
Recent breakthroughs in brain organoid technology have enabled the development of a new in vitro model that promotes significant advancements in the study of nervous system development and diseases1. Sasai and co-workers proposed the idea that differentiated pluripotent cells can form multi-layered organized structures that recapitulate embryonic development when grown in three-dimensional (3D) culture2. Lancaster et al. developed a 3D culture model termed “cerebral organoids” that recapitulate many key features of the human brain in vivo and develop various distinct and interdependent brain regions3. The generation of cerebral organoids depends on the intrinsic ability of pluripotent stem cells (PSCs) to spontaneously self-organize upon precisely timed manipulation of culture conditions even in the absence of external patterning factors3,4,5. Cerebral organoids representing the whole brain have significant advantages over brain region-specific2,6,7,8,9,10,11,12 or extensively patterned organoids13,14,15,16,17 due to their ability to generate a diverse range of brain cells and recapitulate the major events in overall brain development18. Despite the potential of cerebral organoid technology, there are several challenges. Due to the lack of instructive signals during the generation of human cerebral organoids, they recapitulate only some of the earliest stages of human embryonic brain development5 and are not able to mimic the later stages of neurogenesis until extended cultivation for 6–9 months19. Another critical limitation is the extensive cell death in the developing organoids at later stages due to diffusional limitations in oxygen and nutrient transfer4,8.
Several engineering strategies with biomaterials, bioreactors, devices, and genetic modification have been demonstrated to overcome such limitations of current brain organoid culture. For example, synthetic polymer microfilaments enhanced neuroectoderm formation and cortical development by facilitating guided self-organization via neuroepithelium elongation20. In other study, miniaturized spinning bioreactors were tested for improving the dynamic culture of brain organoids, which generated more robust disease models with Zika virus infection8. To increase the oxygen supply, air–liquid interface culture was adapted for cerebral organoids, resulting in improved survival and morphology with extensive axonal outgrowths21. Organ-on-a-chip systems were also employed to improve the oxygen supply to the brain organoids22,23. The vascularization of brain organoids by grafting human brain organoids into the mouse brain or gene editing of vascular transcription factor resulted in progressive neurogenesis with improved neuronal survival24. Despite these recent technical improvements, certain progenitor cells still showed low abundance, and the cytoarchitecture of the basal zones and cortical layers was not complete3,4. Moreover, the current protocols based on spontaneous self-organization have exhibited a significant batch-to-batch variation, which in turn results in poor reproducibility. Therefore, cerebral organoids still need to be improved further for neuronal development, structural maturation, and better electrophysiological functionality, as well as for ensuring consistent organoid quality.
Here, we propose a strategy to engineer human PSC-derived cerebral organoids by reconstituting a 3D brain-mimetic microenvironment with a decellularized human brain tissue-derived brain extracellular matrix (BEM) and dynamic microfluidic systems. BEM can recreate brain-mimetic niches necessary to guide neural and glial differentiation for brain organogenesis, which would likely be deficient in the non-neuronal matrix (e.g. Matrigel)25. The application of microfluidic devices capable of achieving a gravity-driven flow that mimics a fluid flow existing in the cerebrospinal and interstitial spaces can facilitate the oxygen supply and nutrient/waste exchange, leading to a significant reduction of cell death throughout the structure of organoids. Thus, we reason that providing brain-specific extracellular matrix (ECM) cues together with improved nutrient and oxygen exchange will support cell expansion as well as neuronal differentiation and functional maturation, thereby recapitulating prominent features of human embryonic cortical development in a much precise and reproducible manner.
Results
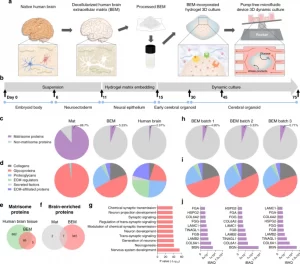
Characterization of a human brain-mimicking 3D hydrogel matrix
A bioengineering platform to improve human brain organoid culture was set up with human brain-mimicking 3D hydrogel and a microfluidic system. Human cerebral organoids were generated from human induced pluripotent stem cells (iPSCs) as described in Lancaster’s protocol (Supplementary Fig. 1)4. When embryoid bodies (EBs) were induced to develop into a neuroepithelial lineage at day 11, they were embedded in a 3D matrix supplemented with human BEM (0.4 mg/ml) (Fig. 1a). Because Matrigel, a common and essential component of the organoid culture, is refractory to the tissue-specific ECM cues that are required by different tissue types26,27, modification of Matrigel-based organoid culture by supplying human BEM would provide enhanced cell growth and more favorable interactions at an early stage of neurogenesis. After four days of culture in BEM-incorporated gel, the organoids were transferred into the microfluidic device under dynamic conditions (Fig. 1a, b). Our microfluidic platform can allow for independent control of the cerebral organoids in much smaller medium volume and precisely controlled medium flow with low fluid shear stress (Supplementary Fig. 2), compared to typical bulk scale bioreactors (e.g. spinner flasks, orbital shakers) which require larger volumes and evoke cell damage due to the high shear stress. With the precisely controlled fluid flow, the effective exchange of oxygen, nutrients, and bioactive molecules in the medium leads to the robust expansion and reduced cell apoptosis at an early stage of organoid development. Consequently, more complex structures with elongated cortical layers would be evident in cerebral organoids.